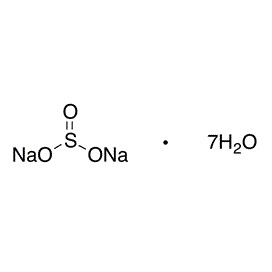
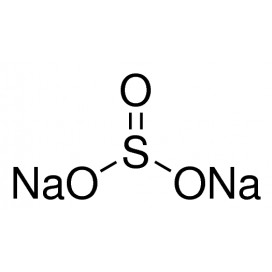
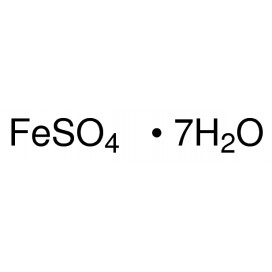
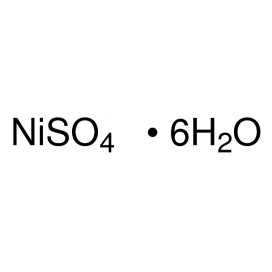
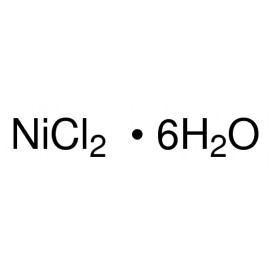
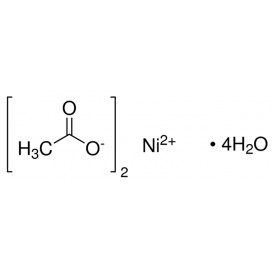
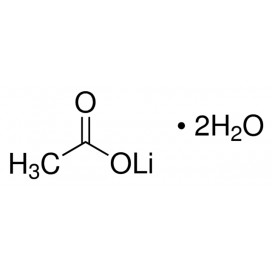
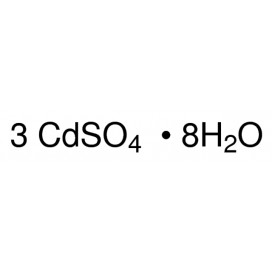
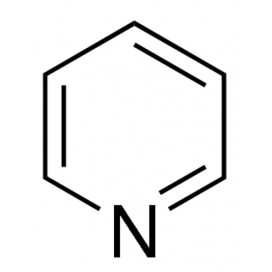
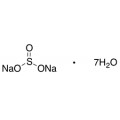
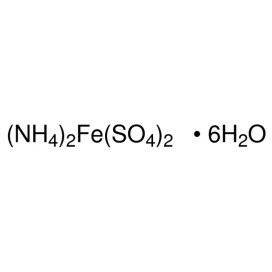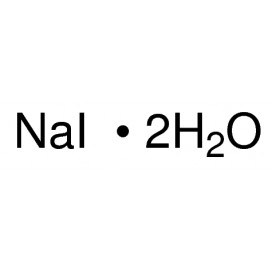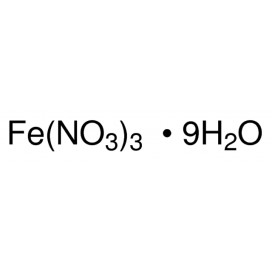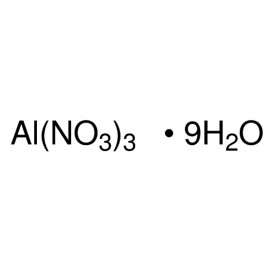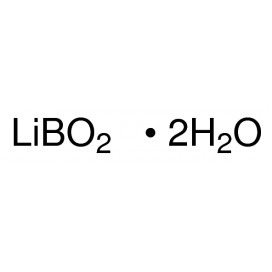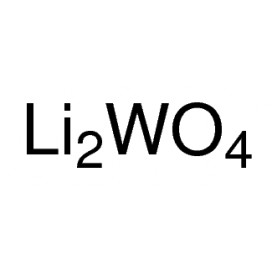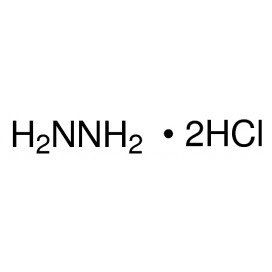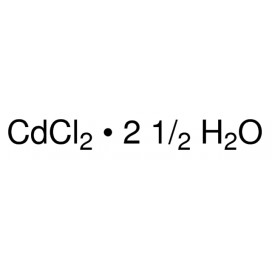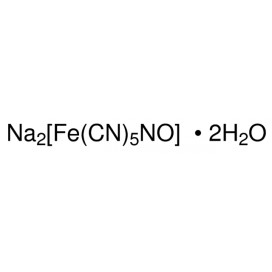Quantity
Total
Product successfully added to your shopping cart
There are 0 items in your cart. There are 0 items in your cart.
Total products
Total shipping (tax incl.) To be determined
Total
Continue shopping Proceed to checkout