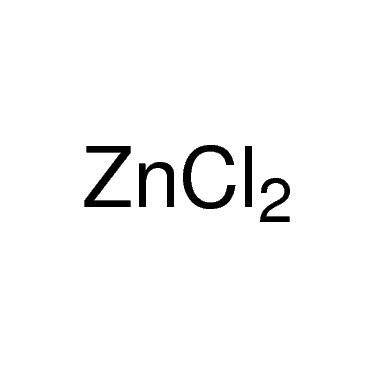
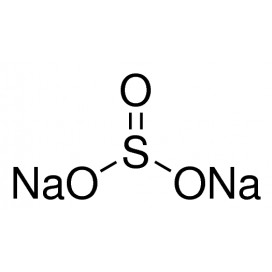
Zinc chloride is the name of chemical compounds with the formula ZnCl2 and its hydrates. Zinc chlorides, of which nine crystalline forms are known, are colorless or white, and are highly soluble in water. ZnCl2 itself is hygroscopic and even deliquescent. Samples should therefore be protected from sources of moisture, including the water vapor present in ambient air. Zinc chloride finds wide application in textile processing, metallurgical fluxes, and chemical synthesis. No mineral with this chemical composition is known aside from the very rare mineral simonkolleite, Zn5(OH)8Cl2·H2O.
Zinc chloride has the ability to react with metal oxides (MO) to give derivatives of the formula MZnOCl2. This reaction is relevant to the utility of ZnCl2 solution as a flux for soldering — it dissolves oxide coatings, exposing the clean metal surface. Fluxes with ZnCl2 as an active ingredient are sometimes called "tinner's fluid". Typically this flux was prepared by dissolving zinc foil in dilute hydrochloric acid until the liquid ceased to evolve hydrogen; for this reason, such flux was once known as "killed spirits". Because of its corrosive nature, this flux is not suitable for situations where any residue cannot be cleaned away, such as electronic work. This property also leads to its use in the manufacture of magnesia cements for dental fillings and certain mouthwashes as an active ingredient.
An early use of zinc chloride (Silzic) was in building carbon skeletons by condensation of methanol molecules. Unsaturated hydrocarbons are the major products, with reaction conditions influencing the distribution of products, though some aromatic compounds were formed. In 1880, it was found that molten zinc chloride catalyses an aromatization reaction generating hexamethylbenzene.
Concentrated aqueous solutions of zinc chloride (more than 64% weight/weight zinc chloride in water) have the interesting property of dissolving starch, silk, and cellulose. Thus, such solutions cannot be filtered through standard filter papers. Relevant to its affinity for these materials, ZnCl2 is used as a fireproofing agent and in fabric "refresheners" such as Febreze. Vulcanized fibre is made by soaking paper in concentrated zinc chloride.
The zinc chloride smoke mixture ("HC") used in smoke grenades contains zinc oxide and hexachloroethane, which, when ignited, react to form zinc chloride smoke, an effective smoke screen.
Ninhydrin reacts with amino acids and amines to form a colored compound “Ruhemann’s purple” (RP). Spraying with a zinc chloride solution forms a 1:1 complex RP:ZnCl(H2O)2, which is more readily detected as it fluoresces better than Ruhemann’s purple.