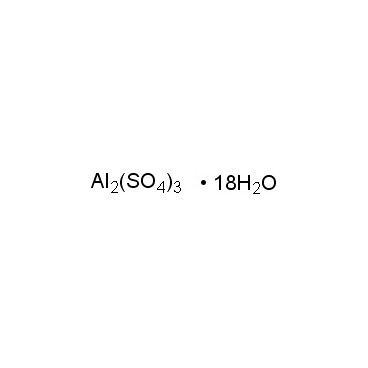
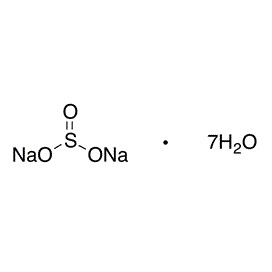
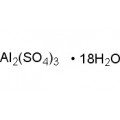Aluminium sulfate is sometimes referred to as a type of alum. Alums are double sulfate salts, with the formula AM(SO4)2·12H2O, where A is a monovalent cation such as potassium or ammonium and M is a trivalent metal ion such as aluminium. The anhydrous form occurs naturally as a rare mineral millosevichite, found e.g. in volcanic environments and on burning coal-mining waste dumps. Aluminium sulfate is rarely, if ever, encountered as the anhydrous salt. It forms a number of different hydrates, of which the hexadecahydrate Al2(SO4)3•16H2O and octadecahydrate Al2(SO4)3•18H2O are the most common. The heptadecahydrate, whose formula can be written as [Al(H2O)6]2(SO4)3•5H2O, occurs naturally as the mineral alunogen.
Aluminium sulfate is used in water purification and as a mordant in dyeing and printing textiles. In water purification, it causes suspended impurities to coagulate into larger particles and then settle to the bottom of the container (or be filtered out) more easily. This process is called coagulation or flocculation. Research suggests that in Australia, aluminium sulfate used this way in drinking water treatment is the primary source of hydrogen sulfide gas in sanitary sewer systems. An improper and excess application incident in 1988 polluted the water supply of Camelford in Cornwall.
When dissolved in a large amount of neutral or slightly alkaline water, aluminium sulfate produces a gelatinous precipitate of aluminium hydroxide, Al(OH)3. In dyeing and printing cloth, the gelatinous precipitate helps the dye adhere to the clothing fibers by rendering the pigment insoluble.
Aluminium sulfate is sometimes used to reduce the pH of garden soil, as it hydrolyzes to form the aluminium hydroxide precipitate and a dilute sulfuric acid solution. An example of what changing the pH level of soil can do to plants is visible when looking at Hydrangea macrophylla. The gardener can add aluminium sulfate to the soil to reduce the pH which in turn will result in the flowers of the Hydrangea turning a different color (blue). The aluminium is what makes the flowers blue; at a higher pH, the aluminium is not available to the plant. In the construction industry, it is used as waterproofing agent and accelerator in concrete. Another use is a foaming agent in fire fighting foam. It is also used in styptic pencils, and pain relief from stings and bites. It can also be very effective as a molluscicide, killing spanish slugs. It is used in dentistry (especially in gingival retraction cords) because of its astringent and hemostatic properties.
Mordants aluminium triacetate and aluminium sulfacetate can be prepared from aluminium sulfate, the product formed being determined by the amount of lead(II) acetate used:
Al2(SO4)3 + 3 Pb(CH3CO2)2 → 2 Al(CH3CO2)3 + 3 PbSO4
Al2(SO4)3 + 2 Pb(CH3CO2)2 → Al2SO4(CH3CO2)4 + 2 PbSO4