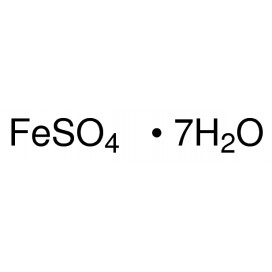
Copper(II) sulfate, also known as cupric sulfate, or copper sulphate, is the inorganic compound with the chemical formula CuSO4(H2O)x, where x can range from 0 to 5. The pentahydrate (x = 5) is the most common form. Older names for this compound include blue vitriol, bluestone, vitriol of copper, and Roman vitriol.
The pentahydrate (CuSO4·5H2O), the most commonly encountered salt, is bright blue. It exothermically dissolves in water to give the aquo complex [Cu(H2O)6]2+, which has octahedral molecular geometry. The structure of the solid pentahydrate reveals a polymeric structure wherein copper is again octahedral but bound to four water ligands. The Cu(II)(H2O)4 centers are interconnected by sulfate anions to form chains.
Anhydrous copper sulfate is a white powder. Copper sulfate pentahydrate is a fungicide. However, some fungi are capable of adapting to elevated levels of copper ions. By mixing a water solution of copper sulfate and a suspension of slaked lime one obtains the Bordeaux mixture, a suspension of copper(II) hydroxide Cu(OH)2 and calcium sulfate, which is used to control fungus on grapes, melons, and other berries.
Cheshunt compound, a mixture of copper sulfate and ammonium carbonate, is used in horticulture to prevent damping off in seedlings. As a non-agricultural herbicide, is it used to control invasive aquatic plants and the roots of plants situated near water pipes. It is used in swimming pools as an algicide. A dilute solution of copper sulfate is used to treat aquarium fishes for parasitic infections, and is also used to remove snails from aquariums. Copper ions are highly toxic to fish, however. Most species of algae can be controlled with very low concentrations of copper sulfate. Copper sulfate inhibits growth of bacteria such as Escherichia coli. Copper(II) sulfate has attracted many niche applications over the centuries. In industry copper sulfate has multiple applications. In printing it is an additive to book binding pastes and glues to protect paper from insect bites; in building it is used as an additive to concrete to provide water resistance and disinfectant qualities. Copper sulfate can be used as a coloring ingredient in artworks, especially glasses and potteries.
Copper sulfate is also used in firework manufacture as a blue coloring agent, but it is not safe to mix copper sulfate with chlorates when mixing firework powders. It corrects copper deficiencies in the soil and animals and stimulates farm animals' growth. In decoration, copper sulfate adds color to cement, metals and ceramic. Some batteries, electrodes and wire contain copper sulfate. It is used in printing ink and hair dye and it creates a green color in fireworks. Several chemical tests utilize copper sulfate. It is used in Fehling's solution and Benedict's solution to test for reducing sugars, which reduce the soluble blue copper(II) sulfate to insoluble red copper(I) oxide. Copper(II) sulfate is also used in the Biuret reagent to test for proteins. Copper sulfate is used to test blood for anemia. The blood is tested by dropping it into a solution of copper sulfate of known specific gravity – blood which contains sufficient hemoglobin sinks rapidly due to its density, whereas blood which does not sink or sinks slowly has insufficient amount of hemoglobin. In a flame test, its copper ions emit a deep green light, a much deeper green than the flame test for barium.
Copper sulfate is employed at a limited level in organic synthesis. The anhydrous salt is used as a dehydrating agent for forming and manipulating acetal groups. The hydrated salt can be intimately mingled with potassium permanganate to give an oxidant for the conversion of primary alcohols. In 2008, the artist Roger Hiorns filled an abandoned waterproofed council flat in London with 75,000 liters of copper sulfate solution. The solution was left to crystallize for several weeks before the flat was drained, leaving crystal-covered walls, floors and ceilings. The work is titled Seizure. Since 2011, it has been on exhibition at the Yorkshire Sculpture Park. Copper sulfate is used to etch zinc or copper plates for intaglio printmaking. It is also used to etch designs into copper for jewelry, such as for Champlevé. Copper sulfate can be used as a mordant in vegetable dyeing. It often highlights the green tints of the specific dyes.