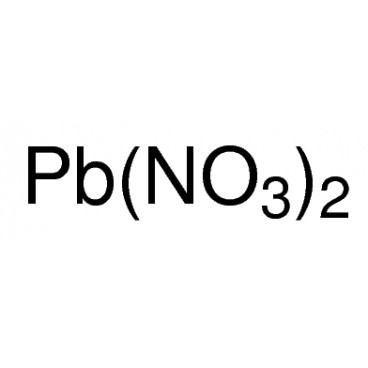Lead(II) nitrate is an inorganic compound with the chemical formula Pb(NO3)2. It commonly occurs as a colourless crystal or white powder and, unlike most other lead(II) salts, is soluble in water.
Known since the Middle Ages by the name plumb dulcis, the production of lead(II) nitrate from either metallic lead or lead oxide in nitric acid was small-scale, for direct use in making other lead compounds. In the 19th century lead(II) nitrate began to be produced commercially in Europe and the United States. Historically, the main use was as a raw material in the production of pigments for lead paints, but such paints have been superseded by less toxic paints based on titanium dioxide. Other industrial uses included heat stabilization in nylon and polyesters, and in coatings of photothermographic paper. Since around the year 2000, lead(II) nitrate has begun to be used in gold cyanidation.
Because of the hazardous nature of lead(II) nitrate, there is a preference for using alternatives in industrial applications. In the formerly major application of lead paints, it has largely been replaced by titanium dioxide. Other historical applications of lead(II) nitrate, such as in matches and fireworks, have declined or ceased as well. Current applications of lead(II) nitrate include use as a heat stabiliser in nylon and polyesters, as a coating for photothermographic paper, and in rodenticides.
On a laboratory scale, lead(II) nitrate provides one of two convenient and reliable sources of dinitrogen tetroxide. By carefully drying lead(II) nitrate and then heating it in a steel vessel, nitrogen dioxide is produced, which dimerizes into the desired compound.
2 NO2 ⇌ N2O4
To improve the leaching process in the gold cyanidation, lead(II) nitrate solution is added. Although a bulk process, only limited amounts (10 to 100 milligrams lead(II) nitrate per kilogram gold) are required. Both the cyanidation itself, as well as the use of lead compounds in the process, are deemed controversial due to the compounds' toxic nature. In organic chemistry, lead(II) nitrate has been used as an oxidant, for example as an alternative to the Sommelet reaction for oxidation of benzylic halides to aldehydes. It has also found use in the preparation of isothiocyanates from dithiocarbamates. Because of its toxicity it has largely fallen out of favour, but it still finds occasional use, for example as a bromide scavenger during SN1 substitution.